Shipping notice
We can only consider requests from institutions, organisations and companies. Due to individual taxes, delivery times and costs depending on your country, you can only request a quote.
Advantages
- Ergonomic design
- Lightweight device with carrying handle
- Intelligent user interface


Product information
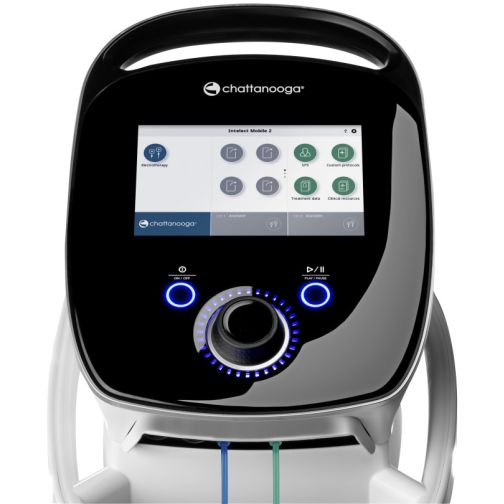
The Intelect® Mobile 2 belongs to the next generation of therapy systems in physical medicine. Its intelligent and intuitive design, features, user-friendliness, and simplicity make the Intelect® Mobile 2 the first choice for the modern practice.
Intelect® Mobile 2 STIM is a 2-channel electrotherapy system. It features 5 current forms: Interferential, VMS, High Voltage, Asymmetrical Biphasic, and Symmetrical Biphasic TENS current. The different current forms can be easily selected through the user-friendly interface and touchscreen. Instructions for electrode placement specific to the chosen treatment area are also displayed.
The Intelect® Mobile 2 is easy to use and set up, thanks to the integrated protocol setting recommendations developed with current clinical practices in mind. The Intelect® Mobile 2 features an easily accessible high-resolution anatomical library, allowing you to visually explain indications and treatment pathways to patients.
Features
- Dimensions: 25.5 x 35.5 x 15 cm
- Weight: 2.9 kg
- High-resolution 7" touchscreen
- Includes instruction according to MPG
- Device cart optional (Item no: T5486)
Included in delivery: Intelect® Mobile 2 STIM
- Intelect® Mobile 2 COMBO
- 4 x carbon electrodes
- 4 x sponges, 6 x 8 cm for carbon electrodes
- Electrode cable channel 1 + 2
- Belt set, 4 straps for electrode fixation
- 4 x Durastick Plus electrodes, 5 x 5 cm
- Power cable
- USB stick
- Printed instruction manual
Technical Data
| Dimensions | |
|---|---|
| Width | 25.5 cm |
| Height | 15 cm |
| Length | 35.5 cm |
| shipping weight | 4 kg |
| Properties & Features | |
| Sports | Physiotherapy |
| Colour | black |
| 2nd Colour | white |
| Weight | 2.9 kg |
| Range of use | therapy |
Scope of Supply
Downloads
Product Safety
-
Medical device according to MDR EU Directive 2017/745Classification according to MDR: Class IIa
More information about the manufacturer
- Manufacturer
-
enovisBötzinger Strasse 90DE - 79111 FreiburgEmail: [email protected]
- Contact partner
-
enovisBötzinger Strasse 90DE - 79111 FreiburgEmail: [email protected]
Sustainability
Any questions about the product?


FAQ | Product questions
There are no product questions yet.
You have an additional question about the product:


